Understanding Your Virginia Soil Test Report
By Gil Medeiros, Former Fairfax Master Gardener
“Don’t guess; soil test!” As master gardeners, we frequently exhort gardeners to do the test, but we are not so helpful in explaining what it means. The reason is that the soil test report itself is an agricultural document designed primarily for farmers and soil scientists. Backyard gardeners struggle to understand the meaning of most of it. Without going into too much chemistry, I will try to impart a working knowledge of the soil test report and what to do with it, simplifying matters for the backyard gardener. Without such adjustments, understanding what to do with your soil test report would not be possible without a degree in chemistry.
We are fortunate in Virginia to have reasonably priced, fast and accurate soil tests. In California and some other states, there is no literature to guide interpretation of state soil test reports because there are no state soil tests.
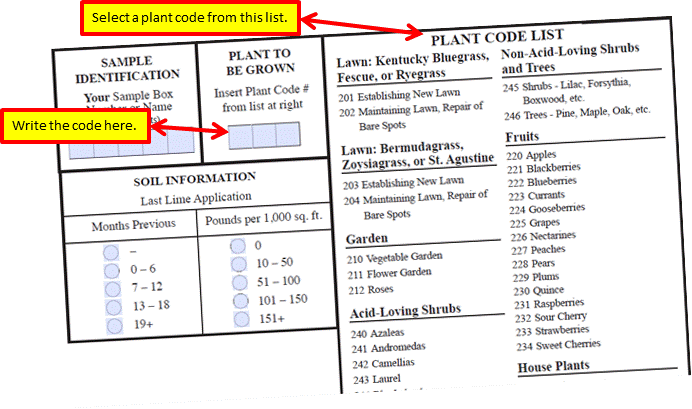
Purpose of the soil test report
The soil test report should help you understand three things:
1. The levels of nutrients available to plants in your soil
Nutrients are the minerals that plants require for growth. The soil test report contains availability levels of these nutrients: phosphorus, potassium, calcium, magnesium, zinc, copper, iron, manganese and boron. The units of measurement are given in either pounds per acre or parts per million.
Why should you care? Even if only one nutrient is deficient, and all the rest are OK, plants will not thrive. This is known in horticulture as Leibig’s Law of the Minimum, and it is why we pay attention to all of the nutrient levels in the report. Fairfax County soil, composed of weathered clay from the gradual erosion of the Piedmont, usually has enough of these nutrients to support plant growth – but not always. The soil test report provides some recommendations about how to correct any nutrient deficiencies that show up in the test.
Although nitrogen is the most important of all plant nutrients, critical for all plant growth, it is NOT included in Virginia soil test results, nor is it included in the reports of most other state testing labs. The reason is that nitrogen levels in soil fluctuate widely due to temperature and rainfall. The lack of consistency makes a nitrogen measurement useless to the gardener. So we handle it this way: We assume there is no nitrogen in the soil at the beginning of the growing season –- the plants used most of it the previous year and what’s left was leached out by rainfall or evaporated as ammonia. If you are an organic gardener, you know this approximation is not exactly right because it does not account for soil nitrogen that is contained in plant and animal waste. Nevertheless, it is fair to assume that you will be replenishing soil nitrogen each year. You will use the fertilizer recommendations in the report and your knowledge of the needs of specific plants -– such as turfgrass or tomatoes –- to determine how much and what kind of nitrogen to add to the soil.
The soil test also does NOT include measurements of toxic elements such as lead or arsenic. They are not plant nutrients. If you are thinking about starting a garden, and you want to know if the soil is contaminated with lead, you must engage a private laboratory to perform that test.
2. The soil pH
This is a measure soil acidity or alkalinity. Most plants like slightly acidic (pH=6.5) or neutral (pH=7) soil.
Why should you care? Soil pH has a strong effect on the availability of soil nutrients to the plants – that makes it a very important measurement. If the pH is too acidic, the availability of nitrogen, phosphorous and potassium — the big three soil nutrients (N-P-K) — is reduced. When the soil is extremely acidic, aluminum, iron and manganese levels may become toxic to the plants. Conversely, iron, zinc and manganese become unavailable when pH becomes too high. This means that the nutrients may be present in the soil, but if the pH is not right, the nutrients may be locked up and unavailable to the plant. Fairfax County soils, like most soils east of the Mississippi, tend to be acidic and usually need periodic applications of lime to stay in the optimum pH range for plant growth (between 6.0 and 7.0).
3. The soil’s ability to retain nutrients and water
This is a measure of how “sticky” the soil is to some nutrients; that is, how well the nutrients are retained in the soil against the leaching effects of rain. There is a direct measure of “stickiness” called Cation Exchange Capacity (CEC). CEC comes with your soil test. There is also an indirect measure: Percent Organic Matter. The latter is an optional measurement that you can order for an additional $4.00. It is usually worth the money.
Why should you care? Think about sand. Pour water into sand, and the water runs right through along with any nutrients dissolved in the water. Soil cannot be fertile if it cannot hold on to nutrients until the plants can use them. Clay in our Fairfax soils “sticks” to and retains nutrients reasonably well, much better than, say, the sandy soils along the coast and some of the kaolin clay soils of the Southeast. Since we have a lot of clay, the CEC is usually in the moderate range of 5 to 12. (The units are milli-equivalents per 100g; this is not important to you.) Organic matter in the soil is even stickier than clay and contributes to the CEC. But organic matter is not typically high in our soils (often under 3 percent). If the CEC is low (typically under 5), we improve the soil’s “stickiness” by adding compost. The increase in organic matter also improves water retention and soil structure.
There are several numbers in the soil test report the home gardener can disregard.
Buffering index
This is part of the calculation the Soil Testing Lab makes to advise you of how much lime to add to soil to correct an acidic pH. You do not have to pay attention to it.
Base saturation
This is useful to soil scientists and farmers who attempt to manage soil nutrition in reference to an “ideal” model. These numbers have no practical application for the home gardener.
Soluble salts
This is an optional measurement for an extra $2.00. It is almost never needed by the home gardener unless there is a suspected problem with either over-fertilization or run-off from salts used to melt ice. Rainfall is the cure; soluble salts leach out of the soil over time.
The information in the reports and how to interpret it.
The reports have three sections.
1. Sample history
This information is provided by the person submitting the sample. You can ignore any category that you did not provide. For example, SMU-1 stands for Soil Map Unit 1.The SMUs and the Yield Estimate and the Productivity Group are pieces of information provided by farmers and do not have value for home gardeners.
2. Lab Test Results
Your measured nutrient availability values are here. Phosphorus (P), potassium (K), calcium (Ca) and magnesium (Mg) are expressed as pounds per acre. Since none of us are soil scientists, the numerical results are less important than the Ratings: VH, H, H-, M+, M, M-, L+,L, or L-. As you may surmise, the H stands for high; M stands for medium, and L stands for low. Consider that anything below medium will require corrective action through the addition of fertilizer. Anytime the measurement is between medium and high, the plants will benefit from addition of some fertilizer. If the nutrient is in the very high range, do not add it; the plant will not take it up. These considerations are addressed in the Fertilizer and Limestone Recommendations (described below).
Zinc (Zn), manganese (Mn), copper (Cu), iron (Fe), and boron (B) are reported as parts per million. These are the micronutrients. Again, we are less concerned with the numbers than the ratings. Here, the Soil Test Lab uses a simple scale: sufficient (SUFF) or deficient (DEF). Consider that anything in the deficient range will require corrective action. Normally this is done in the home landscape simply by addition of compost, particularly compost made in part from animal manure.
Pay close attention to the numerical values for Soil pH. A pH of 6.5 is right for turfgrass and most garden plants, but there are exceptions. Blueberries like an acidic pH around 5. Blue hydrangeas need a similar pH to produce blue-colored flowers. Azaleas and rhododendrons like a pH of 5.5. In contrast, lavender likes neutral to slightly basic soil just above a pH of 7. One size does not fit all.
As a rule of thumb, the Cation Exchange Capacity (CEC) is OK above 5 and very good above 15. If it is below 5, the addition of compost to the soil is warranted to improve “stickiness” for nutrients and water retention. This will also help soil structure.
Organic Matter (%) is an optional measure for an additional $4.00. This is a useful measurement. For most soils, anything below 3 percent should be corrected by adding compost. Five percent is about right up to about 10 percent for most garden situations. Measurements in excess of 10 percent indicate there is too much organic matter in the soil. At these levels, the soil becomes bog-like. It retains too much water and not enough air and can become unhealthy for plants.
3. Fertilizer and limestone recommendations
These are computer generated, based on the measurement levels and what you have indicated will be grown in that soil. They tell you exactly what corrective actions or improvements need to be taken. Pay close attention to them.
Three samples of soil test reports
Report Sample 1
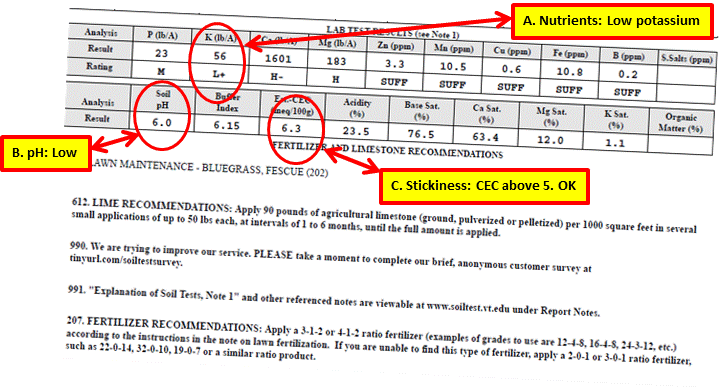
This is a soil test result I received in 2015 for the back lawn section of my yard. Let’s look at the data.
Actions as a Result of Test
A. All of the nutrient levels measured at medium or above or sufficient, except for potassium at L+ (Low plus). Any measurement below medium or Insufficient needs to be addressed through some kind of fertilization. Therefore, the potassium level needed to be raised. This did not come as a total surprise since I had fertilized my lawn with Milorganite for the previous three years, and Milorganite (5-2-0) contains no potassium. So the potassium from lawn fertilizers I had applied more than three years ago was depleted. I made two applications of a lawn maintenance fertilizer containing potassium at one of the recommended ratios.
B. The pH was too acidic. I applied ground limestone at the recommended rate of 50 pounds per 1000 square feet.
C. Stickiness
The level is above 5. No corrective action was necessary. However, I will consider aerating the soil and top dressing with compost in the future.
Report Sample 2
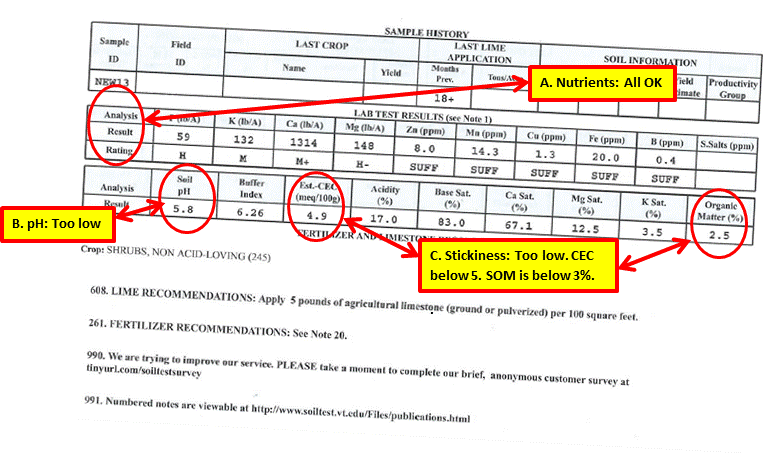
This test report is for an area where I wanted to put a new planting bed of perennials in the spring of 2013.
A. All of the nutrient levels measured at medium or above or sufficient. No corrective was required.
B. At 5.8 the pH was too acidic. I applied ground limestone at the recommended rate of 5 pounds per 100 square feet.
C. The CEC level was below 5, and Soil Organic Matter level was below 3 percent. The measurements are in agreement and indicate addition of compost to the soil. Based upon the total area of the bed, I rototilled 10 bags of compost into the soil. After planting, I added a layer of hardwood mulch, which will continue to add organic matter to the soil.
Report Sample 3
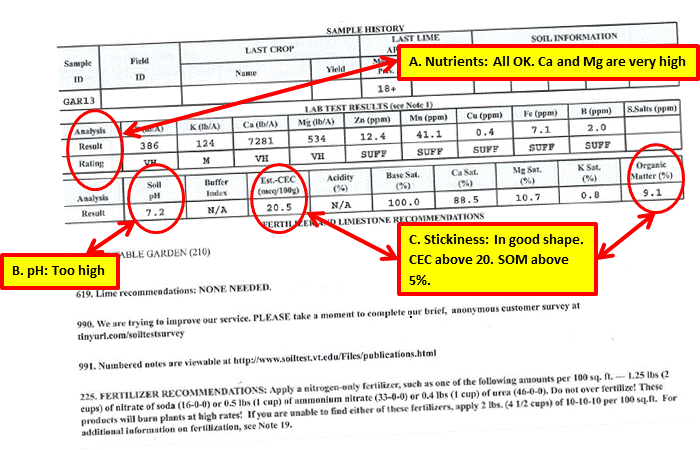
This test report is for my vegetable garden. It was taken in early spring of 2013.
Actions as a Result of Test
A. All of the nutrient levels measured at medium or above or sufficient. No corrective action was required.
B. At 7.28, the pH was too high. I have not applied lime since doing the test. With calcium levels very high, I was not concerned about a calcium deficiency causing blossom end rot in tomatoes and peppers.
C. Stickiness: The CEC level was above 20, and Soil Organic Matter level was well above 3 percent. The measurements are in agreement and are the result of consistent application of compost annually over many years. I have continued compost application at the same rate since the test.
Armed with this knowledge, you should be able to amaze your friends and neighbors by pointing out things in their soil test reports that they never realized. Give it a try.
References
• Explanation of Soil Tests, Virginia Cooperative Extension, Publication 452-701
• How to Convert an Inorganic Fertilizer Recommendation to an Organic One, Wayne McLaurin
& Water Reeve, University of Georgia Extension
• Interpreting Your Soil Test Results, University of Massachusetts Extension
• Soil Test Recommendations for Virginia, R. O. Maguire & S. E. Heckendor, Virginia Cooperative
Extension