Phenomenal pH Phacts
By Ray Novitske, Fairfax Master Gardener
Some of us may remember the pink and blue litmus paper test from our days in chemistry class. This test determined if a substance was an acid or a base, but not the magnitude. pH measures just that — how acid or alkaline a substance is.
What is pH?
The abbreviation pH stands for potenz hydrogen, a German term meaning “power of hydrogen” or “potential of hydrogen.” (Elements on the periodic table are often represented by a capital letter, thus the capital H.) Often pH is translated to English as “potential hydrogen.” The term is German because, as the story goes, the pH scale was first developed by a German brewer to determine the acid concentration of the beer he was brewing. Other references mention the concept of pH being introduced by a Danish chemist named Sørensen in 1909, in a French-speaking lab where pH stood for puissance hydrogen, meaning ”hydrogen power.”
 Hydrogen without an electron has a positive charge due to the missing negative charge of its electron and the remaining positive charge of its nucleus. In this state, it is known as a hydrogen ion. Simply stated, a concentration of these hydrogen ions higher than neutral water equals more acidity, while a lower concentration means more alkalinity.
Hydrogen without an electron has a positive charge due to the missing negative charge of its electron and the remaining positive charge of its nucleus. In this state, it is known as a hydrogen ion. Simply stated, a concentration of these hydrogen ions higher than neutral water equals more acidity, while a lower concentration means more alkalinity.
If water is assigned a value of 7.0 as the starting point, substances with measured pH values less than 7.0 are considered acid (lots of hydrogen ions), and those with values above 7.0 are considered alkaline. As an example, extremely acidic lemon has a pH of 2.5 and vinegar, 3.0. Extremely alkaline ammonia is 11.1, and lime 12.0.
Measurement values are not a straight-line relationship but a logarithmic scale. With the pH formula, each point on the scale represents a tenfold increase, so a soil pH of 5.0 is 10 times more acidic than a soil pH of 6.0.
Why pH is important in gardening
With the chemistry and math explanations behind us, let’s look at how pH affects our gardens. pH simply affects how well plants take up nutrients from the soil. Plants take up essential nutrients that are dissolved in water. Most nutrients can dissolve in a range from pH 6.0 to 7.5. When soil is too acidic or too alkaline, some nutrients don’t dissolve well, so they will not be taken up by the roots.
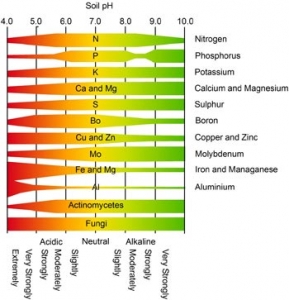
Availability of nutrients at various pH levels
In highly acidic soils potassium, calcium, phosphorus and magnesium become tied up and unavailable. pH values below 4.5 create toxicity in plants because of an increase in the availability of aluminum, iron and manganese.
pH can also affect the activity of beneficial microorganisms in the soil. Bacterial decomposition is hindered by strongly acidic soil, preventing the breakdown of organic matter. This can tie up nitrogen in the accumulating organic matter instead of allowing the nitrogen to be released in a soluble form for plant use. And strongly acidic soils cause many herbicides to lose effectiveness.
How pH in soils is determined
 In the Eastern U.S., with heavy rainfall and forest cover, soils are naturally more acidic. Rainfall is slightly acidic, and it leaches out some neutralizing compounds, leaving more acids. And, heavy leaf cover on the ground produces more acidic soils. Oak leaves are most acidic, but they acidify the soil only modestly. The pH of leaf mulch must be considered when you use this material in your gardens. In more desert-like climates with higher temperatures, as in the South and West, evaporation leads to salt accumulations, giving soil more alkalinity.
In the Eastern U.S., with heavy rainfall and forest cover, soils are naturally more acidic. Rainfall is slightly acidic, and it leaches out some neutralizing compounds, leaving more acids. And, heavy leaf cover on the ground produces more acidic soils. Oak leaves are most acidic, but they acidify the soil only modestly. The pH of leaf mulch must be considered when you use this material in your gardens. In more desert-like climates with higher temperatures, as in the South and West, evaporation leads to salt accumulations, giving soil more alkalinity.
Heavy limestone below the surface increases pH of the soil. Areas in Florida and parts of the country with limestone below the surface have this problem. Soils around concrete foundations of new homes tend to be more alkaline because calcium compounds in the concrete leach into the soil. This makes it difficult to grow plants that favor highly acidic conditions.
Altering pH to suit plant requirements
Most plants grow happily in slightly acidic or neutral soils, in a pH range of 6.0 to 7.0, the same range in which most nutrients easily dissolve in water. There are exceptions: blueberries, azaleas, rhododendrons and hollies prefer more acidic conditions. Native woodland plants tend to be comfortable in areas with more acidic soil, such as in deciduous or pine forests where the leaves and needles affect soil structure.
Adding organic matter tends to buffer pH, bringing it closer to neutral. Changing an acidic soil by adding lime is the most frequent cure gardeners use. Clay soils, because of their tendency to hold more water than sandy soils and not leach out, may require more lime than sandy soils to improve the pH, even if both start at the same pH level. In our area, soil improved with lime should require repeated applications every 3 to 4 years simply because of the tendency of soil to revert to its natural pH.
Lime acts slowly, so adding it to improve soil pH should be done in the fall for results in the spring. Effective liming agents such as calcium carbonate or dolomitic lime bind with hydrogen ions and remove them from the soil, which in turn reduces acidity. Wood ashes are also effective at raising pH because of their high calcium carbonate content. Limit their use to 25 pounds per 1000 square feet because, being very fine, they are fast acting.
Decreasing soil pH can be accomplished through the use of elemental sulfur. Products are available specifically to use for acid-loving plants, although most contain fertilizers, too. Decreasing the pH of highly alkaline soils is very difficult. There is no way to permanently lower pH in soils formed with a high calcium content such as limestone or soils severely impacted by construction materials. In these circumstances, select plants that are tolerant of high pH levels.
Home test kits are available to measure the pH of your soil. A soil test performed by a lab or by Virginia Tech will, however, give you not only a pH measurement but also recommendations to remedy deficiencies. The recommendations are based on the plants you grow and the type of soil you have (sandy, clay or something in between.) Do-it-yourself kits do not provide detailed solutions to soil problems.
It takes a bit of chemistry and math to know how pH works, but a basic understanding of its importance and function will help you keep it in the correct range and give you better results in the garden.
References
• Soil Test Note #1 – Explanation of Soil Tests, Rory Maguire, Steve Heckendorn, Virginia Cooperative
Extension, Virginia Tech
• Soil pH: What it Means, State University of New York College of Environmental Science and Forestry
• Soil pH and Salinity, University of Arizona
• Soil pH and the Home Landscape or Garden, Rao Mylavarapu and Christine Wiese, University of
Florida Extension
• pH of Freshly Fallen Leaves, Forests of the Central Appalachians Project
• Understanding Soil pH, Michigan State University Extension
• Think like a plant when measuring soil pH, Oregon State University Extension
• Soil pH Affects Nutrient Availability, University of Maryland Extension
• Understanding pH, Rodale’s Organic Life, Rodale Inc.